Life sciences companies are facing tougher obstacles in bringing new therapies to market than ever before. With an average drug approval process taking up to 15 years and billions of dollars to complete, new technological advancements are being leveraged to help manage the growing clinical, regulatory, and commercial needs necessary to accelerate time to market.
Veeva Systems is one of those leading technologies, offering a cloud-based solution that helps hundreds of life sciences companies accelerate critical business functions, from R&D through commercial. Veeva Vault, in particular, serves as a content management platform that can uniquely manage both content (such as approved marketing collateral) and data ranging from study results, safety/efficacy data, and much more.

Integration is key to transforming life sciences operations
To power all of these respective business functions, life sciences companies need to pull in data siloed across a vast network of CROs, clinical systems like CTMS and EDCs, real-world evidence data, legacy/ERP systems and hundreds of other sources. With an average business transaction – such as sourcing trial data results – crossing more than 35 different technology systems, the challenges of accessing siloed data can lead to life sciences IT teams managing a messy network of tightly coupled systems and applications.
This approach can make it difficult to integrate critical data from new partners and CROs in a timely fashion, leading to duplicative efforts, poor data quality, and an increased risk of trial non-compliance. According to Cutting Edge Information, 72% of studies run more than one month behind schedule, and such delays can cost sponsors between $600,000 and $8 million for each day that a trial delays a product’s development and launch.
MuleSoft offers a new approach to connecting data, devices, and systems to Veeva
With MuleSoft, life sciences IT teams are embracing a new approach to connectivity to meet the scale and speed necessary to solve their integration challenges. From global organizations to innovative upstarts, life sciences companies are powering digital transformation in the industry through our market-leading Anypoint Platform and innovative approach to connectivity.
Per the clinical example above, Anypoint Platform provides an enterprise-grade integration solution and an API-led approach that champions the reuse of assets, facilitating timely onboarding and data sharing across CROs, investigators and other entities involved in your clinical trial operations to improve collaboration and reduce the risk of protocol deviation.
MuleSoft has helped life sciences companies surface data across different sources into Veeva applications with reusable APIs that help accelerate the timetable of additional projects. With MuleSoft, life sciences IT teams can change the speed of project delivery and drive digital transformation.
MuleSoft’s new Veeva Vault Connector accelerates time to value for Veeva customers
With the new MuleSoft Veeva Vault Connector, life sciences companies can easily integrate Veeva Vault with other business applications and systems. The ability to easily move data in and out of Veeva Vault improves business agility and allows companies to accelerate their development efforts. Veeva customers can leverage this new connector to integrate Veeva Vault with any data or system, without needing to develop or maintain custom code.
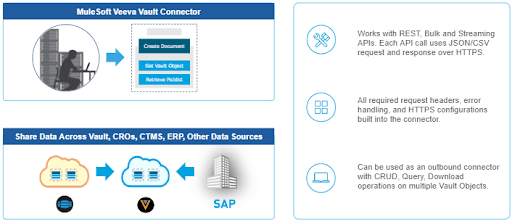
The Veeva Vault Connector can be used in Mule applications as an outbound connector with CRUD (Create, Retrieve, Update, Delete), Query, Export operations on multiple Vault Objects.
Benefits of the new connector include:
- Providing seamless integrations between Veeva Vault and other applications for greater efficiency and compliance.
- Reducing the need to develop and maintain custom integration code.
- Allowing Veeva customers to integrate data silos much easier and faster to facilitate critical business initiatives from R&D, safety, commercial and others.
Transform your clinical operations with MuleSoft and Veeva
With the new MuleSoft Veeva Vault Connector, current Veeva customers can surface data across any of the suite of Veeva Vault products available — supporting business needs across clinical data management, operations, regulatory, safety, medical, and commercial needs.

Interested in learning more? Register for our upcoming webinar to see how this new Veeva Vault Connector can securely integrate Veeva Vault with CTMS, ERP, MDM, legacy third-party systems, and business applications to power mission-critical initiatives while ensuring compliance every step of the way.